Which of the following best characterizes a hydrogen bond. Hydrogen bond is weak force between atoms in a molecule but is of enormous ad_2 Related.

Solved Question 6 Which Of The Following Best Describes Chegg Com
55 of the class answered correctly.
. London dispersion forces are the weakest intermolecular force. Which statement explains covalent bonding between two atoms in a molecule. The overall dipole for HFC-23 is 165 D.
The correct answer. B when electrons are shared between two atomsGroups of atoms held together by covalent bonds are called. As a Rule of Thumb they are weaker than covalent and ionic intramolecular bonds but stronger than most dipole-dipole interactions.
T is a weak and temporary electrical attraction between two polar molecules. It is a strong chemical bond formed by the sharing of valence electrons. A hydrogen bond is when a polar hydrogen containing molecule interacts with another polar molecule to.
First molecules has hydrogen attached to a highly electronegative atom NOF. The true statement about the hydrogen bond is c. Hydrogen bonds are a strong type of dipole-dipole interaction.
Which statement best describes hydrogen bonding. Select all the statements that correctly describe intermolecular hydrogen bonding. 2 The electropositive hydrogen atom in one molecule and an electropositive atom in another molecule experience attraction.
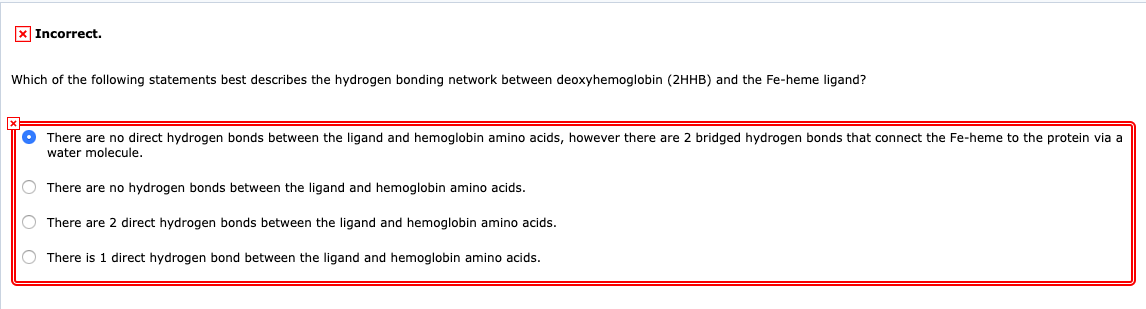
Which of the following statements best describes the hydrogen bonding network between deoxyhemoglobin 2HHB and the Fe-heme ligand. It forms only between nonpolar molecules. AThere are no direct hydrogen bonds between the ligand and hemoglobin amino acids however there are 2 bridged hydrogen bonds that connect the Fe-heme to the protein via atomic oxygen.
Van der waals forces are the strongest force between h2 molecules and between nh3 molecules. There are no hydrogen bonds between the ligand and hemoglobin amino acids. 35 _____ A Weak interactions that become significant when molecules are very close together B Very strong bonding between cations and anions C Electrostatic attraction between charged areas of adjacent molecules D Very strong bonding from the sharing of electrons between atoms.
A hydrogen bond is when hydrogen shares electrons with another element creating a new molecule through a covalent bond. The electronegative hydrogen atom in one molecule and an electropositive atom in another molecule experience attraction. Choice B Water has an overall negative charge which allows it to easily dissolve nonpolar substances like glucose.
There are 2 direct hydrogen bonds between the ligand and hemoglobin amino acids. 35 Which of the following best describes hydrogen bonding. Due to hydrogen bonding water is more dense as a liquid than a solid.
Hydrogen bonding is the strongest force between h2 molecules and between nh3 molecules. Dispersion and dipole-dipole interactions occur among HFC-134a molecules. Which of the following statements best describes the hydrogen bonding network between deoxyhemoglobin 2HHB and the Fe-Heme ligand.
2 The electropositive hydrogen atom in one molecule and an electropositive atom in another molecule experience attraction. Choice C Water molecules are polar consisting of two partially positive hydrogen atoms and one partially negative oxygen atom. The O 2 Lewis structure has a double bond between two oxygen atomsAccording to the octet rule oxygen atoms need to bond twice.
Which statement best describes the intermolecular forces between h2 molecules and nh3 molecules in the liquid phase. There are no hydrogen bonds between the ligand and hemoglobin amino acids. 3 on a question.
A hydrogen bond is when a polar hydrogen containing molecule interacts with another polar molecule to make a weak non-covalent bond c. It is formed only between different molecules intermolecular. Which of the following statements best describes the hydrogen bonding network between deoxyhemoglobin 2HHB and the Fe-heme ligand.
3 The electronegative hydrogen atom in one. Hydrogen bonding occurs in all of these materials EXCEPT LiH How does a coordinate covalent bond differ from a standard covalent bond. Which statement best describes the energy change as bonds are formed and broken in this reaction.
Which statement best describes hydrogen bonding1 point 1 The electronegative hydrogen atom in one molecule and an electropositive atom in another molecule experience attraction. There are 2 direct hydrogen bonds between the ligand and hemoglobin amino acids. There are two requirements for hydrogen bonding.
The electronegative hydrogen atom in one molecule and an. Which statement best describes the intermolecular forces between molecules of HFC-134a. A The forming of the H-Cl bond releases energy b The forming of the H-Cl bond absorbs energy c The breaking of the H-H bond releases energy d The breaking of the Cl-Cl bond releases energy.
1 The electronegative hydrogen atom in one molecule and an electropositive atom in another molecule experience attraction. The electropositive hydrogen atom in one molecule and an electropositive atom in another molecule experience attraction. In the source of the electrons shared Which statement describes the strength of London dispersion forces.
Which statement best describes hydrogen bonding. BThere are 2 direct hydrogen bonds. A hydrogen bonding is a type of covalent bond that forms between a hydrogen and an electronegative atom b hydrogen bonding is an intermolecular force existing between two molecules that have hydrogens bonded to an O N or F c hydrogen bonding is important to many biological.
The most common incorrect answer is that hydrogen bonding was present.
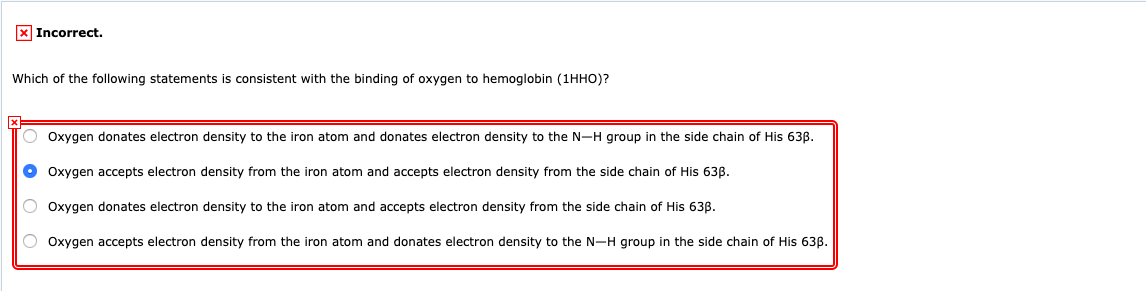
Solved X Incorrect Which Of The Following Statements Best Chegg Com

Hydrogen Bonding Chemistry For Non Majors

Solved X Incorrect Which Of The Following Statements Best Chegg Com
0 Comments